- Home
- About
- Portfolio
Crush the Match – Medical School and Residency Platform
Food¢ense – Curbing Childhood Obesity and Food Waste
HealthStack – Shared and Jailed HIPAA Hosting $50
Marta Care – Let Us Help When You Can’t
MD Idea Lab – We Build Prototypes for Doctors
Nervcell – The Healthcare Web Browser
Patient Keto – Personalized Keto Medicine and Telehealth
SwipeChart – Rapid EMR Interface
Treatment Scores – Quantifying the Science of Medicine
Treatments – Diagnosed. Now What?
VIDRIO – Google Glass and EMR Interface
- Blog
- Contact
- Home
- Warp Core Health
- Blog
- Mitochondria
Category: Mitochondria
Explaining How Glucose and Ketones Become ATP: Why Ketosis and Fat Adaptation Boost Health
- November 9, 2024
- Stephen Fitzmeyer, MD
- No Comments
Fuel Sources
When it comes to energy, the body has two primary fuel sources: glucose and ketones. While glucose is often the default source, especially on a typical high-carb diet, ketones become the primary fuel under low-carb or fasting conditions. Ketosis, the metabolic state where the body produces ketones from fat, isn’t just about an alternative fuel source; it also offers several health benefits, including weight loss, improved metabolic health, reduced cardiovascular disease risk, and fat adaptation. Let’s dive into how glucose and ketones each produce ATP and why ketosis is a preferred metabolic state for overall health.
ATP: The Body’s Essential Energy Currency
ATP (adenosine triphosphate) fuels virtually every function in our bodies, from muscle contractions to cognitive activities. The body produces ATP by metabolizing nutrients through complex biochemical pathways, using glucose and ketones as primary fuels. Interestingly, ketones produce more ATP per molecule than glucose, making them an efficient energy source, especially beneficial during ketosis.
Pathway 1: Converting Glucose to ATP
Under normal dietary conditions, glucose is the body’s main energy source. Here’s how glucose becomes ATP through a process called cellular respiration:
Step 1: Glycolysis
- Breakdown of Glucose: In the cytoplasm, glucose is split into two molecules of pyruvate.
- Initial ATP Yield: Glycolysis yields 2 ATP per glucose molecule and produces NADH (an electron carrier for later stages).
Step 2: Pyruvate to Acetyl-CoA
- Conversion in the Mitochondria: Pyruvate enters the mitochondria and is converted to acetyl-CoA, releasing CO₂ and generating more NADH.
Step 3: Citric Acid Cycle (Krebs Cycle)
- Energy Harvesting: Acetyl-CoA enters the citric acid cycle, where it is broken down to produce NADH and FADH₂, which fuel ATP production in the next step.
Step 4: Electron Transport Chain (ETC)
- Main ATP Production: NADH and FADH₂ donate electrons to the ETC, which powers ATP synthase to produce ATP as protons flow back across the mitochondrial membrane.
ATP Yield: Glucose metabolism produces approximately 30-32 ATP per molecule, though it also creates byproducts that can contribute to oxidative stress.
Pathway 2: Converting Ketones to ATP – A More Efficient Pathway
When carbohydrate intake is low, the body shifts to using fat for fuel, producing ketones in the liver. Ketones offer several advantages over glucose, not least of which is their higher energy yield.
Step 1: Ketogenesis in the Liver
- Fat Conversion to Ketones: In the liver, fatty acids are converted into ketones (mainly beta-hydroxybutyrate and acetoacetate) through ketogenesis.
- Transport to Tissues: These ketones are released into the bloodstream and delivered to cells for energy.
Step 2: Ketone Conversion to Acetyl-CoA
- Entry into the Mitochondria: Once inside the cell, ketones are converted back to acetyl-CoA, entering the mitochondria to power the next steps in ATP production.
Step 3: Citric Acid Cycle and Electron Transport Chain
- High ATP Yield: Ketones enter the citric acid cycle, generating NADH and FADH₂, which support the electron transport chain.
Why Ketones Produce More ATP: Ketones, specifically beta-hydroxybutyrate and acetoacetate, produce ATP efficiently but with unique metabolic advantages. When fully oxidized, beta-hydroxybutyrate yields approximately 21.5 ATP per molecule, and acetoacetate provides about 19 ATP, giving a combined total of about 40.5 ATP per pair of ketone molecules. In comparison, one molecule of glucose goes through glycolysis, the citric acid cycle, and the electron transport chain to produce around 30-32 ATP in total. Though glucose produces a comparable amount of ATP, ketones offer metabolic stability, lower oxidative stress, and more efficient energy production in low-carb states, making them an effective and sustainable fuel source, especially during fasting or ketogenic conditions.
Why Ketosis and Fat Adaptation Are Beneficial
The body’s ability to switch from glucose to ketones isn’t just a backup mechanism; it provides significant health benefits. This metabolic flexibility is key for weight loss, metabolic health, and cardiovascular protection. Here’s why ketosis, and the fat adaptation that comes with it, is so advantageous:
- Weight Loss: Ketosis and fat adaptation (when the body becomes efficient at using fat for fuel) are incredibly effective for weight loss. Since the body can tap into stored fat for energy, people in ketosis burn more fat while maintaining stable energy levels. Ketosis also suppresses hunger hormones, making it easier to stick to a calorie deficit.
- Metabolic Syndrome Management: Ketosis helps combat metabolic syndrome by reducing insulin resistance. In ketosis, blood sugar levels are more stable, and the body becomes less reliant on insulin to manage glucose levels. This stability can reverse metabolic syndrome symptoms, which often include high blood pressure, high triglycerides, and abdominal obesity, reducing the risk of type 2 diabetes.
- Reduced Cardiovascular Disease Risk: Ketosis supports cardiovascular health by reducing triglyceride levels and increasing HDL (good) cholesterol. Additionally, ketones produce fewer reactive oxygen species (ROS), meaning less oxidative stress and inflammation, both of which contribute to heart disease. The anti-inflammatory effects of ketosis make it beneficial for long-term cardiovascular protection.
- Fat Adaptation for Enhanced Energy: Fat adaptation is the process where the body becomes efficient at using fat and ketones as its primary energy source. Once fat-adapted, the body can seamlessly access stored fat for sustained energy, which is especially useful for endurance activities and fasting. People who are fat-adapted experience stable energy without the spikes and crashes associated with glucose, making fat adaptation a valuable state for consistent physical and cognitive performance.
- Brain Health and Focus: Ketones readily cross the blood-brain barrier, providing a steady fuel for brain cells. Many people report improved mental clarity, focus, and cognitive endurance in ketosis due to the brain’s ability to efficiently use ketones as fuel, especially when blood sugar levels are low.
Embracing Metabolic Flexibility
By optimizing metabolic pathways to use both glucose and ketones efficiently, the body achieves metabolic flexibility, the ability to switch seamlessly between fuel sources. This flexibility promotes balanced energy levels, reduces cravings, and supports long-term health.
In Summary
- Glucose Pathway: The body breaks down glucose through glycolysis and the citric acid cycle, yielding around 30-32 ATP. However, glucose metabolism can lead to oxidative stress and relies on stable blood sugar levels for consistent energy.
- Ketone Pathway: Ketones are produced in the liver from fatty acids and yield more ATP per molecule than glucose. This efficiency, along with reduced byproducts, makes ketones a cleaner and more sustainable fuel, especially during ketosis and fat adaptation.
Ketosis not only provides a high-yield, steady energy source but also supports fat loss, metabolic syndrome improvement, and cardiovascular health. By fostering metabolic flexibility, ketosis allows the body to switch efficiently between glucose and ketones, supporting optimal energy levels and overall health.
This article emphasizes how ketones provide a cleaner, higher-yielding fuel source that supports fat loss, improved metabolic health, and cardiovascular protection. For those looking to optimize their health, embracing ketosis and fat adaptation offers a powerful way to achieve lasting vitality and metabolic balance.
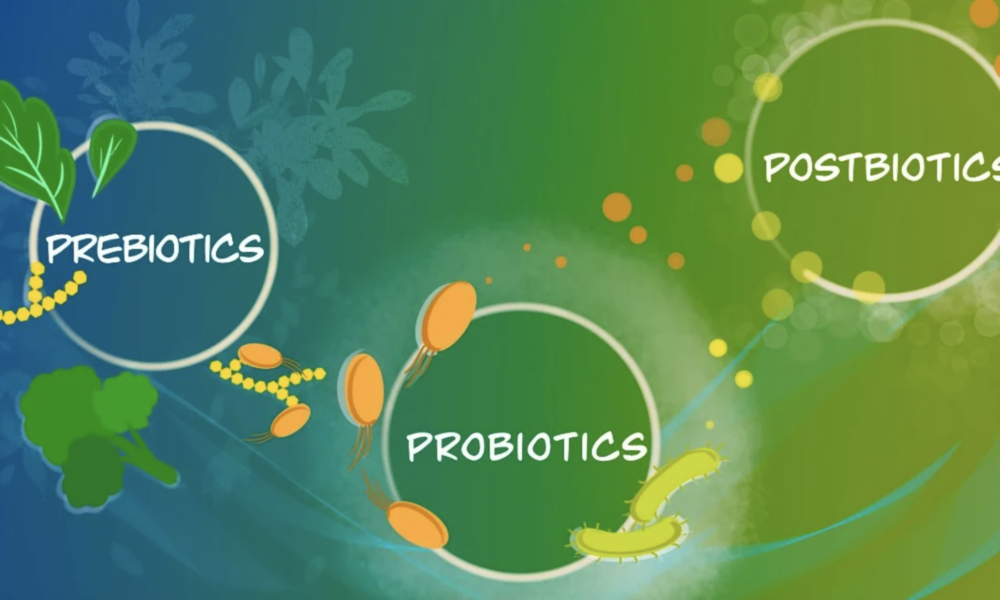
Exploring the Role of Prebiotics, Probiotics, and Postbiotics in a Keto Diet
- May 15, 2024
- Stephen Fitzmeyer, MD
- No Comments
Introduction: The ketogenic diet, known for its low-carbohydrate and high-fat approach, has gained popularity for weight loss and metabolic benefits. While specific keto foods do not naturally contain probiotics, incorporating fermented foods into the diet can provide probiotic benefits. Additionally, understanding the concepts of prebiotics and postbiotics can further enhance gut health and overall well-being. In this article, we will delve into the significance of prebiotics, probiotics, and postbiotics within the context of a ketogenic diet.
Probiotic Sources in a Keto Diet: Fermented vegetables, such as sauerkraut and kimchi, can be included in a ketogenic diet to introduce beneficial bacteria. Look for options without added sugars or high-carb ingredients. Some low-carb, unsweetened, full-fat yogurts containing live and active cultures, like Lactobacillus acidophilus and Bifidobacterium strains, can also be suitable. Kefir, whether made from milk or non-dairy alternatives like coconut milk or water, provides a range of beneficial bacteria and yeast strains. Additionally, naturally fermented pickles and miso can be considered, but portion control is essential due to their carbohydrate content.
Understanding Prebiotics: Prebiotics are non-digestible fibers that serve as food for beneficial gut bacteria. While not providing direct probiotic benefits, they help nourish and support the growth of beneficial bacteria in the gut. Some prebiotic-rich keto-friendly foods include non-starchy vegetables like leafy greens, broccoli, cauliflower, asparagus, and garlic. These can be incorporated into meals to promote a healthy gut microbiota while maintaining ketosis.
The Role of Postbiotics: Postbiotics are the byproducts or metabolites produced by probiotic bacteria during fermentation. They include substances like short-chain fatty acids (SCFAs), enzymes, vitamins, and organic acids. SCFAs, such as butyrate, acetate, and propionate, have been extensively studied for their health benefits. They can regulate the immune system, support gut barrier function, and have anti-inflammatory and antimicrobial effects. While research on postbiotics is still emerging, they show promise as a way to reap the benefits of probiotics without consuming live bacteria.
Conclusion: Incorporating prebiotic-rich foods, fermented vegetables, and certain types of yogurt, kefir, pickles, and miso can provide probiotic benefits while following a keto diet. These foods can help nourish the gut microbiota and promote a healthy balance of beneficial bacteria. Additionally, understanding the role of prebiotics and postbiotics adds depth to gut health management. Prebiotic-rich foods like non-starchy vegetables support the growth of beneficial bacteria, while postbiotics offer potential health benefits without the need for live bacteria. As always, it is important to consult with healthcare professionals or registered dietitians for personalized advice based on individual dietary needs and health considerations. By incorporating prebiotics, probiotics, and understanding postbiotics, individuals can optimize their gut health while following a keto diet and reap the potential benefits for overall well-being.
Author: Dr. Stephen Fitzmeyer, M.D.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Mitochondrial Metabolism: An Essential Regulator of Adipose Tissue, Metabolic Health, Inflammation, and Brain Function
- September 15, 2023
- Stephen Fitzmeyer, MD
- No Comments
By Stephen Fitzmeyer, MD
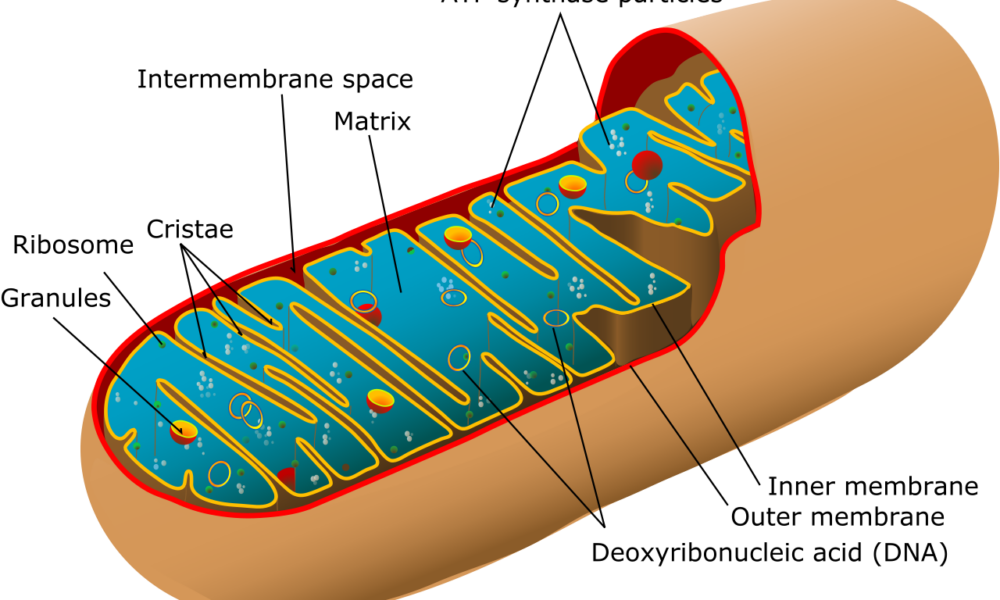
Mitochondria, often referred to as the powerhouses of the cell, play a crucial role in various aspects of human physiology. Beyond their well-known role in energy production, emerging research has shed light on the intricate relationship between mitochondrial metabolism and adipose tissue development and function. Moreover, recent discoveries have highlighted the impact of mitochondrial metabolism on metabolic health, inflammation, and even brain function. Understanding these connections could pave the way for new therapeutic strategies in tackling obesity, metabolic disorders, and neurodegenerative diseases.
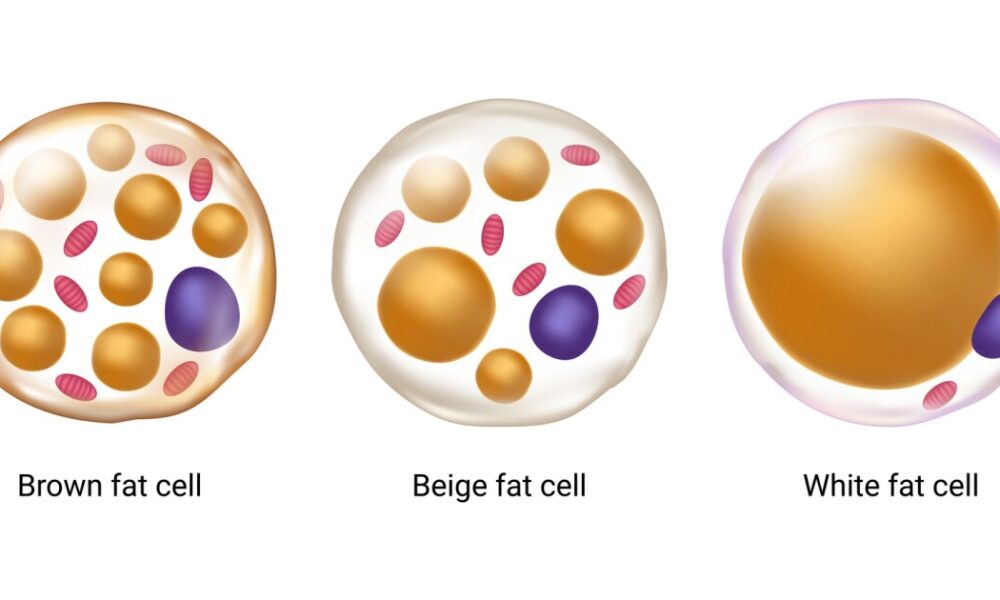
Adipose tissue, commonly known as fat, was once perceived as an inert energy storage depot. However, it is now recognized as a dynamic and metabolically active organ that influences whole-body homeostasis. Adipose tissue consists of two main types: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT primarily stores energy in the form of triglycerides, while BAT dissipates energy through thermogenesis. Both types of adipose tissue are influenced by mitochondrial metabolism, albeit in different ways.
In WAT, mitochondrial metabolism has been found to regulate adipogenesis, the process by which precursor cells differentiate into mature adipocytes. Studies have shown that impaired mitochondrial function leads to dysfunctional adipocyte differentiation and altered adipose tissue development. Furthermore, mitochondrial dysfunction in WAT has been linked to insulin resistance, a hallmark of metabolic disorders such as obesity and type 2 diabetes.
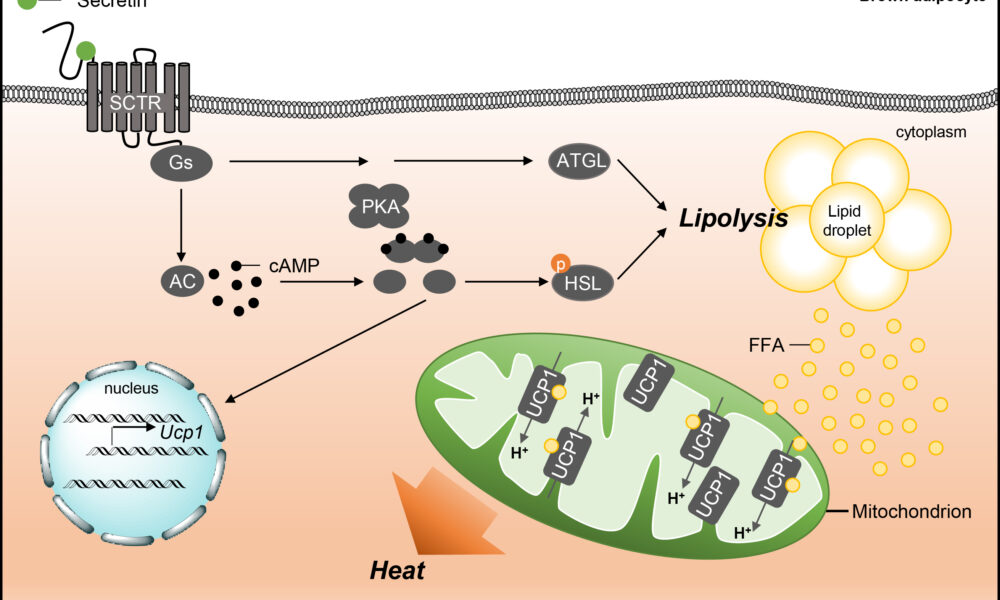
On the other hand, BAT is enriched with mitochondria and possesses a high capacity for oxidative metabolism. Brown adipocytes express a protein called uncoupling protein 1 (UCP1), which uncouples oxidative phosphorylation from ATP synthesis, resulting in the generation of heat. This unique characteristic of BAT is essential for maintaining body temperature and regulating energy expenditure. Emerging evidence suggests that impaired mitochondrial metabolism in BAT contributes to obesity and metabolic dysfunction. Conversely, enhancing mitochondrial function in BAT has been proposed as a potential therapeutic strategy to combat obesity and associated metabolic disorders.
Mitochondrial metabolism not only influences adipose tissue development and function but also plays a pivotal role in metabolic health and inflammation. Dysfunctional mitochondria can lead to an imbalance in cellular energy metabolism, resulting in the accumulation of toxic metabolites and the generation of reactive oxygen species (ROS). Excessive ROS production contributes to oxidative stress and chronic low-grade inflammation, which are closely associated with obesity, insulin resistance, and cardiovascular diseases. Inflammation disrupts normal adipose tissue function and can further exacerbate metabolic dysfunction.
Furthermore, recent studies have highlighted the impact of mitochondrial metabolism on brain health and function. The brain is a highly energy-demanding organ, and mitochondrial dysfunction has been implicated in various neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases. Impaired mitochondrial function in the brain can lead to reduced energy production, compromised neuronal activity, and increased vulnerability to oxidative stress and inflammation. Therefore, maintaining mitochondrial health in the brain is crucial for preserving cognitive function and preventing neurodegeneration.
The intricate interplay between mitochondrial metabolism, adipose tissue development, metabolic health, inflammation, and brain function underscores the importance of understanding these relationships in a holistic manner. Targeting mitochondrial dysfunction may hold promise for therapeutic interventions aimed at improving metabolic health, combating obesity, and even mitigating neurodegenerative diseases.
In conclusion, mitochondrial metabolism is a key regulator of adipose tissue development and function. It influences both white and brown adipose tissues, impacting metabolic health, inflammation, and even brain function. Exploring the molecular mechanisms underlying these connections could provide valuable insights into the pathogenesis of obesity, metabolic disorders, and neurodegenerative diseases. Ultimately, this knowledge may open doors to novel therapeutic strategies that target mitochondrial function, empowering individuals to take control of their metabolic well-being and combat the growing burden of obesity and associated diseases. By promoting mitochondrial health and optimizing adipose tissue function, we may pave the way for a healthier future.
It is evident that mitochondria play a multifaceted role in our bodies, extending far beyond their traditional association with energy production. Their influence on adipose tissue development and function, metabolic health, inflammation, and brain function highlights their significance in maintaining overall physiological balance.
As researchers continue to delve into the intricate mechanisms that govern mitochondrial metabolism, new therapeutic avenues may emerge. Targeted interventions aimed at enhancing mitochondrial function could potentially revolutionize the treatment of metabolic disorders, including obesity, insulin resistance, and neurodegenerative diseases.
Moreover, advancements in our understanding of mitochondrial metabolism may lead to the identification of novel biomarkers for early detection and risk assessment of these conditions. This could enable personalized interventions and interventions at an earlier stage, with the potential to halt or reverse disease progression.
However, it is important to acknowledge that the complexities of mitochondrial metabolism and its interactions with various bodily systems require further investigation. Ongoing research is needed to unravel the underlying mechanisms and to validate the potential therapeutic strategies that target mitochondrial function.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Unlocking the Power of Fat: Understanding Brown Fat, White Fat, and Ketones in Metabolism
- September 1, 2023
- Stephen Fitzmeyer, MD
- No Comments
By Stephen Fitzmeyer, MD
Introduction:
The human body is a complex machine that relies on various mechanisms to maintain energy balance and regulate metabolism. In recent years, significant research has been conducted to understand the different types of fat and their roles in energy storage, thermogenesis, and overall metabolic health. Additionally, the impact of ketones, particularly beta-hydroxybutyrate (BHB), on uncoupling and thermogenesis in white fat has emerged as a fascinating area of study. This article aims to delve into the fascinating world of brown fat, white fat, and the influence of ketones on fat metabolism.
Brown Fat: The Furnace of Heat Generation
Brown fat, also known as brown adipose tissue (BAT), is a specialized form of fat that plays a crucial role in thermogenesis. Unlike white fat, which primarily stores energy, brown fat is densely populated with mitochondria that contain a unique protein called uncoupling protein 1 (UCP1). UCP1 enables the uncoupling of electron transport and ATP synthesis, diverting energy towards heat production. By activating brown fat, the body can generate heat and maintain body temperature, making it an important component in combating hypothermia and regulating energy expenditure.
White Fat: Beyond Energy Storage
White fat, or white adipose tissue (WAT), is the more abundant type of fat in the human body and is primarily associated with energy storage. White fat cells store excess energy in the form of triglycerides, which can be released when energy is needed. However, recent research has shown that white fat can exhibit properties similar to brown fat through a process called browning or beiging. Browning involves the activation of UCP1 in white fat cells, leading to increased thermogenesis and energy expenditure. This discovery has opened up new possibilities for harnessing the potential of white fat in weight management and metabolic health.
Ketones: Fueling the Metabolic Fire
Ketones, specifically beta-hydroxybutyrate (BHB), have garnered attention for their impact on fat metabolism and uncoupling in white fat. During periods of low carbohydrate availability, such as fasting or adherence to a ketogenic diet, the body produces ketones as an alternative fuel source. Ketones can enhance uncoupling in white fat by increasing UCP1 expression, improving mitochondrial function, and activating specific signaling pathways. This process promotes thermogenesis and energy expenditure in white fat cells, potentially contributing to weight loss and metabolic health benefits associated with ketogenic diets.
Metabolic Flexibility and Health Implications
Understanding the intricate interplay between brown fat, white fat, and ketones provides insights into metabolic flexibility and its impact on health. Activating brown fat and promoting browning of white fat can increase energy expenditure, potentially assisting in weight management and combating obesity. Additionally, the utilization of ketones as an alternative fuel source offers metabolic advantages, such as improved mitochondrial function and uncoupling in white fat, which may have implications for metabolic health and conditions such as diabetes and cardiovascular disease.
Conclusion:
The exploration of brown fat, white fat, and the influence of ketones on fat metabolism has unveiled exciting possibilities for understanding energy balance, thermogenesis, and metabolic health. The ability to activate brown fat, induce browning of white fat, and harness the power of ketones could provide new avenues for managing weight, improving metabolic health, and combating metabolic disorders. As research in this field continues to evolve, we are gaining a deeper understanding of the intricate mechanisms that govern our metabolism and pave the way for innovative strategies in promoting a healthier future.
Author: Stephen Fitzmeyer, M.D.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Harnessing the Power of Probiotics: Exploring Mitochondrial Uncoupling and its Benefits
- May 7, 2023
- Stephen Fitzmeyer, MD
- No Comments
Introduction: Probiotics have gained considerable attention for their potential health benefits, especially in the context of gut health. However, recent studies have revealed an intriguing connection between probiotics and mitochondrial uncoupling, a process that holds promise for various health benefits. In this article, we will explore the role of probiotics in mitochondrial uncoupling and delve into the potential advantages it offers.
Understanding Mitochondrial Uncoupling: Mitochondria are the powerhouses of our cells, responsible for energy production. Normally, energy production occurs through a tightly regulated process called oxidative phosphorylation, where adenosine triphosphate (ATP) is generated. However, mitochondrial uncoupling refers to the disruption of this process, leading to the dissipation of energy as heat instead of ATP production. This phenomenon is facilitated by a protein called uncoupling protein 1 (UCP1) and is primarily found in brown adipose tissue (BAT) and beige fat cells.
The Link Between Probiotics and Mitochondrial Uncoupling: Recent studies have demonstrated that certain probiotic strains can influence mitochondrial uncoupling and enhance the activity of UCP1. Specifically, probiotics like Bifidobacterium breve, Lactobacillus plantarum, and Akkermansia muciniphila have shown potential in promoting the browning of white adipose tissue, leading to increased thermogenesis and energy expenditure. These probiotics can modulate the gut microbiota composition and promote the release of specific metabolites, such as short-chain fatty acids (SCFAs), that play a role in mitochondrial uncoupling.
The Benefits of Probiotic-Induced Mitochondrial Uncoupling: Mitochondrial uncoupling, induced by probiotics, offers several potential benefits:
- Increased energy expenditure: By promoting thermogenesis and energy dissipation as heat, mitochondrial uncoupling can potentially boost overall energy expenditure, which may be beneficial for weight management and metabolic health.
- Improved glucose metabolism: Studies have suggested that probiotic-induced mitochondrial uncoupling may improve glucose metabolism and insulin sensitivity, which could be particularly advantageous for individuals with type 2 diabetes or metabolic disorders.
- Enhanced fat oxidation: Mitochondrial uncoupling can stimulate the breakdown of stored fat and enhance fat oxidation, potentially aiding in weight loss and reducing body fat.
- Regulation of inflammation: Probiotics that induce mitochondrial uncoupling have been associated with reduced inflammation and improved gut barrier function, which may have positive implications for various inflammatory conditions.
Conclusion: The emerging research on probiotic-induced mitochondrial uncoupling highlights a fascinating link between gut health and metabolic processes. Probiotics, such as Bifidobacterium breve, Lactobacillus plantarum, and Akkermansia muciniphila, show potential in promoting mitochondrial uncoupling and unlocking its associated benefits, including increased energy expenditure, improved glucose metabolism, enhanced fat oxidation, and regulation of inflammation. However, it is essential to note that further research is needed to fully understand the mechanisms and long-term effects of probiotic-induced mitochondrial uncoupling.
As our understanding of the gut-brain axis and the intricate connections within our bodies continues to grow, harnessing the power of probiotics for mitochondrial uncoupling opens up new avenues for potential health interventions. As always, consulting with healthcare professionals or specialists in the field can provide personalized advice and guidance for incorporating probiotics and optimizing their benefits in relation to mitochondrial uncoupling.
Author: Dr. Stephen Fitzmeyer, M.D.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Recent Posts
- Protected: Warp Core Health: Building a Custom AI Model for Transforming Healthcare
- The Intersection of Healthcare, AI, Clinical Informatics, and Machine Learning
- Accessing Siloed EMR Systems with FHIR: Connecting to Multiple EMRs
- How AI and Informatics Are Transforming Healthcare
- How AI Can Transform Healthcare Applications
Categories
- ApoB
- Artificial Intelligence
- Autophagy
- Biochemistry
- Biomedical Informatics
- Biostatistics
- Blood Glucose
- CAC
- Carbs
- CCD
- CDA
- Clinical Informatics
- Coding Bootcamp
- Coronary Artery Disease
- COVID-19
- Cybersecurity
- Data Science
- Diabetes
- Diet
- EHS
- EMR
- Epidemiology
- Evidence Based Medicine
- Fats
- FHIR
- Fiber
- Generative AI
- Global Health
- Health Administration
- Health Informatics
- Health IT
- HIPAA
- HL7
- Hyperglycemia
- Hypoglycemia
- ICD 10
- Intermittent Fasting
- Ketogenic Diet
- Machine Learning
- Macronutrients
- MCT Oil
- Metabolic Health
- Metabolic Syndrome
- Minerals
- Mitochondria
- MySQL
- Neurology
- Nutritional Ketosis
- Nutritional Neurology
- Nutritional Psychiatry
- PHP
- PHR
- Programming
- Prompt Engineering
- Proteins
- Prototypes
- Public Health
- Python
- Recipes
- Sleep Health
- Stroke
- Uric Acid
- Vegan and Vegetarians
- Vitamin D
- Vitamin K2
- Vitamins