- Home
- About
- Portfolio
- Crush the Match – Medical School and Residency Platform
- Food¢ense – Curbing Childhood Obesity and Food Waste
- HealthStack – Shared and Jailed HIPAA Hosting $50
- Marta Care – Let Us Help When You Can’t
- MD Idea Lab – We Build Prototypes for Doctors
- Nervcell – The Healthcare Web Browser
- Patient Keto – Personalized Keto Medicine and Telehealth
- SwipeChart – Rapid EMR Interface
- Treatment Scores – Quantifying the Science of Medicine
- Treatments – Diagnosed. Now What?
- VIDRIO – Google Glass and EMR Interface
- Blog
- Contact
Month: June 2023
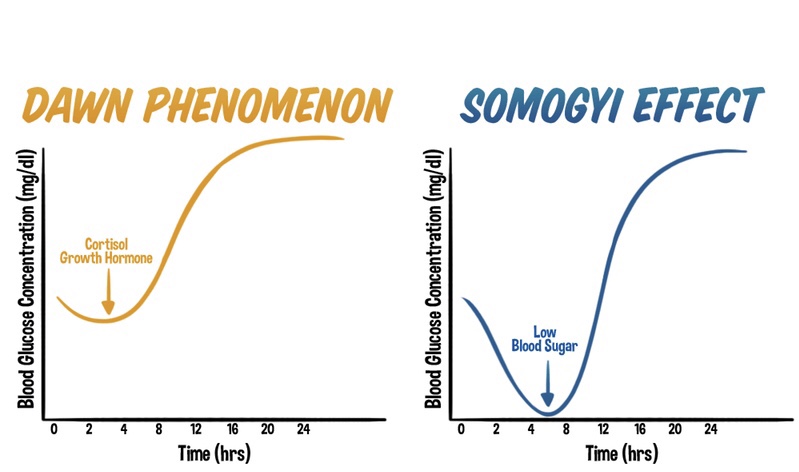
Unveiling the Differences: The Dawn Phenomenon vs. The Somogyi Effect in Diabetes Management
By Stephen Fitzmeyer, MD
Introduction:
Diabetes management encompasses various challenges, including understanding and addressing the intricacies of blood glucose fluctuations. Two phenomena that often perplex individuals with diabetes and healthcare professionals are the dawn phenomenon and the Somogyi effect. While both involve abnormal blood glucose levels, these phenomena differ in their timing, triggers, underlying mechanisms, and management strategies. In this article, we delve into these distinctions to shed light on the unique characteristics of the dawn phenomenon and the Somogyi effect in diabetes management.
The Dawn Phenomenon: An Early Morning Rise in Blood Glucose
The dawn phenomenon is a well-known phenomenon observed in individuals with diabetes, characterized by an abnormal rise in blood glucose levels during the early morning hours, typically before waking up. Hormonal changes play a significant role in triggering this phenomenon. Increased release of hormones such as cortisol, growth hormone, and glucagon during the early morning hours leads to insulin resistance and stimulates gluconeogenesis. As a result, blood glucose levels rise without any preceding hypoglycemia.
The Somogyi Effect: Rebound Hyperglycemia Following Nocturnal Hypoglycemia
In contrast, the Somogyi effect involves a rebound hyperglycemia following a period of nocturnal hypoglycemia. This phenomenon occurs when blood glucose levels drop too low during the night, often due to excessive insulin administration or inadequate carbohydrate intake before bedtime. Nocturnal hypoglycemia triggers a counterregulatory response in the body, resulting in the release of hormones such as glucagon, cortisol, and growth hormone. These hormones stimulate gluconeogenesis and glycogenolysis, leading to a rebound rise in blood glucose levels during the morning or throughout the day.
Distinguishing Factors: Timing, Triggers, and Underlying Mechanisms
One of the primary distinctions between the dawn phenomenon and the Somogyi effect lies in their timing and triggers. The dawn phenomenon occurs during the early morning hours, driven by natural hormonal changes, while the Somogyi effect occurs as a response to nocturnal hypoglycemia.
Underlying mechanisms also differ between the two phenomena. The dawn phenomenon involves overactive gluconeogenesis as a contributing factor, as the liver produces glucose from non-carbohydrate sources. In contrast, the Somogyi effect encompasses a complex interplay of factors, including the release of counterregulatory hormones that stimulate both gluconeogenesis and glycogenolysis.
Management Strategies:
Effective management of the dawn phenomenon and the Somogyi effect requires tailored approaches based on their unique characteristics.
Managing the dawn phenomenon involves adjusting insulin regimens, specifically optimizing basal insulin doses during the early morning hours. Lifestyle modifications, including regular exercise, a balanced diet, and adequate sleep, can also aid in stabilizing blood glucose levels.
The management of the Somogyi effect requires identifying patterns of nocturnal hypoglycemia through consistent blood glucose monitoring. Adjusting insulin doses, timing, or types can prevent hypoglycemia and subsequent rebound hyperglycemia. Ensuring sufficient carbohydrate intake before bedtime and maintaining consistent sleep patterns are essential strategies in managing the Somogyi effect.
Conclusion:
Understanding the distinctions between the dawn phenomenon and the Somogyi effect is crucial in diabetes management. While both phenomena involve abnormal blood glucose fluctuations, their timing, triggers, underlying mechanisms, and management strategies differ significantly. Healthcare professionals play a vital role in recognizing these differences and tailoring individualized care plans to optimize blood glucose control. By comprehending the unique characteristics of the dawn phenomenon and the Somogyi effect, individuals with diabetes can work with their healthcare teams to effectively manage these phenomena and achieve improved overall well-being.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Unraveling the Dawn Phenomenon: Understanding Gluconeogenesis in Type 1 Diabetes
By Stephen Fitzmeyer, MD
Introduction:
The dawn phenomenon is a well-known phenomenon observed in individuals with type 1 diabetes, characterized by an abnormal rise in blood glucose levels during the early morning hours, even in the absence of food intake. It has been a subject of scientific curiosity and investigation for many years. While the exact cause of the dawn phenomenon remains unclear, one hypothesis suggests that overactive gluconeogenesis may play a significant role in its manifestation. In this article, we delve into the relationship between the dawn phenomenon and gluconeogenesis in type 1 diabetes to shed light on this intriguing phenomenon.
Understanding the Dawn Phenomenon:
To comprehend the dawn phenomenon, it is essential to grasp the concept of gluconeogenesis. Gluconeogenesis is a natural process in which the liver produces glucose from non-carbohydrate sources, such as amino acids and glycerol. This metabolic pathway is crucial in maintaining blood glucose levels during periods of fasting or prolonged exercise.
In individuals with type 1 diabetes, who lack insulin production, the dawn phenomenon is believed to occur due to the combined effect of several factors. During the late night and early morning hours, hormones such as cortisol, growth hormone, and glucagon are released in higher amounts. These hormones work together to increase insulin resistance and stimulate hepatic gluconeogenesis. The elevated blood glucose levels observed in the morning are thought to be a consequence of these hormonal changes.
Role of Gluconeogenesis in the Dawn Phenomenon:
Gluconeogenesis is regulated by a complex interplay of hormonal and metabolic factors. Under normal circumstances, insulin suppresses gluconeogenesis, primarily by inhibiting the release of glucagon and promoting glucose uptake in peripheral tissues. However, in type 1 diabetes, the absence of insulin disrupts this balance, resulting in uncontrolled gluconeogenesis.
Research has suggested that the dawn phenomenon may be associated with overactive gluconeogenesis. Studies have shown increased levels of hepatic glucose production during the early morning hours in individuals with type 1 diabetes experiencing the dawn phenomenon. This excessive glucose production can contribute to the elevated blood glucose levels observed upon waking.
Alternative Factors:
While overactive gluconeogenesis is one plausible explanation for the dawn phenomenon, it is important to note that other factors may also contribute to its occurrence. The release of counterregulatory hormones, such as cortisol and growth hormone, may promote hepatic glucose output, leading to increased blood glucose levels. Additionally, alterations in circadian rhythms and overnight hypoglycemia followed by a rebound effect might also contribute to the dawn phenomenon.
Clinical Implications:
Understanding the mechanisms underlying the dawn phenomenon is crucial for effective diabetes management. Several strategies can help mitigate its impact. Adjusting insulin regimens, particularly by optimizing basal insulin doses during the early morning hours, can help counteract the excessive hepatic glucose production. Additionally, lifestyle modifications such as regular exercise, a balanced diet, and adequate sleep may aid in maintaining stable blood glucose levels.
Conclusion:
While the dawn phenomenon in individuals with type 1 diabetes remains a subject of ongoing research, overactive gluconeogenesis appears to be one of the contributing factors. The hormonal changes that occur during the early morning hours, coupled with the absence of insulin, disrupt the delicate balance of glucose regulation. Further research is needed to unravel the intricate mechanisms involved in the dawn phenomenon fully. By gaining a deeper understanding of this phenomenon, healthcare professionals can develop more effective strategies to manage blood glucose levels and improve the overall well-being of individuals living with type 1 diabetes.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
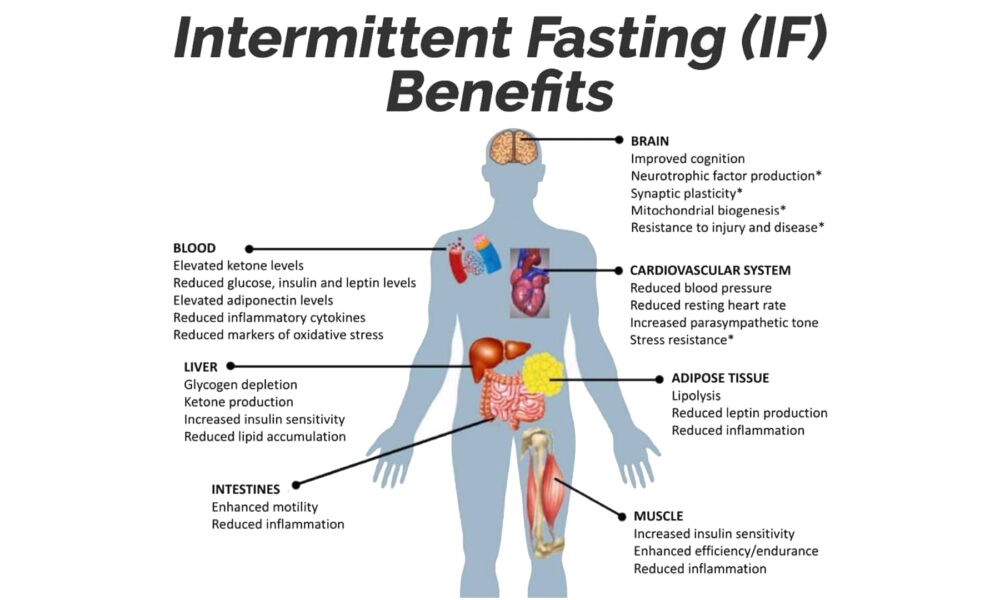
Intermittent Fasting: The Key to Metabolic Health, Empowered by the Ketogenic Diet for Satiety, Hunger Reduction, and Craving Control
By Stephen Fitzmeyer, MD
Introduction
In the realm of metabolic health, one strategy has risen above the rest: Intermittent Fasting (IF). When coupled with the Ketogenic (Keto) diet, IF becomes an unrivaled approach that unlocks the full potential of satiety, hunger reduction, and cravings control. By harnessing the power of fasting, IF paves the way for optimal metabolic function, while the Keto diet amplifies these benefits, leading to improved overall health and well-being.
Intermittent Fasting: A Metabolic Game Changer
Intermittent Fasting has garnered widespread acclaim due to its profound impact on metabolic health. Rather than focusing solely on what you eat, IF centers on when you eat, establishing periods of fasting interspersed with designated eating windows.
At the core of IF lies its ability to promote metabolic flexibility. By depriving your body of constant food intake, it becomes adept at tapping into stored fat as an alternative energy source. This metabolic switch leads to weight loss, increased insulin sensitivity, and reduced inflammation.
Satiety and Hunger Reduction: The Role of IF
One of the key advantages of IF is its capacity to enhance satiety and curb hunger. During fasting periods, your body turns to its fat stores for fuel, facilitating fat burning and weight loss. However, the benefits extend far beyond shedding pounds.
IF effectively regulates hunger and fullness hormones, such as ghrelin and leptin, which influence appetite. With consistent practice, these hormones rebalance, resulting in reduced hunger and decreased cravings. By allowing your body ample time between meals, IF equips you with a newfound sense of control over your eating habits.
The Power of the Ketogenic Diet
Enter the Ketogenic diet, a low-carbohydrate, high-fat approach that synergizes remarkably with IF. By drastically reducing carbohydrate intake and increasing healthy fat consumption, the Keto diet promotes nutritional ketosis—a metabolic state where your body primarily relies on fat for energy.
The Keto diet is a satiety powerhouse. Healthy fats take longer to digest, keeping you feeling full and satisfied for extended periods. This phenomenon effectively curbs hunger, reduces cravings, and prevents the energy crashes associated with high-carbohydrate diets.
IF and Keto: The Dynamic Duo for Metabolic Health
When Intermittent Fasting and the Ketogenic diet join forces, a metabolic transformation occurs. IF acts as the catalyst, priming your body for efficient fat burning, while the Keto diet ensures that fat becomes the primary fuel source.
By following a Ketogenic diet within your designated eating window, you not only maintain a state of ketosis but also heighten the feeling of satiety. The combined approach effectively reduces hunger and cravings, making it easier to adhere to your dietary goals and achieve optimal metabolic health.
Conclusion
Intermittent Fasting is the key to unlocking metabolic health, and when paired with the Ketogenic diet, it becomes an unbeatable strategy for satiety, hunger reduction, and craving control. IF enhances your body’s ability to tap into stored fat for energy and regulates hunger hormones, while the Keto diet amplifies these effects through increased fat consumption.
Embrace Intermittent Fasting as your metabolic ally and leverage the Ketogenic diet as the perfect complement. Together, they offer a path to sustainable weight loss, improved insulin sensitivity, and enhanced overall well-being. Experience the transformative power of IF and Keto, and embrace a life of metabolic vitality.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
From Keto to Carnivore: Decoding Low Carb Diets for Ultimate Health and Vitality
By Stephen Fitzmeyer, MD
Introduction:
In the quest for improved health and weight management, numerous dietary approaches have gained popularity. Among the most well-known are the low carb diets, including the ketogenic diet (keto) and the carnivore diet. However, it is important to understand the subtle nuances and benefits of each variation, as well as other popular low carb diets such as the Paleo, Mediterranean, and Standard American Diet (S.A.D.). In this article, we will explore the differences and benefits of these dietary choices, shedding light on the variables that make each one unique.
The Ketogenic Diet (Keto):
The ketogenic diet is a low carb, high fat diet that encourages the body to enter a state of ketosis. By significantly reducing carbohydrate intake and increasing fat consumption, the body shifts from using glucose as its primary fuel source to using ketones. This metabolic state has been associated with several benefits, including weight loss, improved insulin sensitivity, and increased mental clarity. Additionally, keto has shown promise in managing epilepsy and certain neurological disorders.
The Carnivore Diet:
At the other end of the spectrum lies the carnivore diet, which emphasizes exclusively animal products and eliminates plant-based foods entirely. This ultra-low carb, high fat, and high protein approach aims to mimic the dietary patterns of our ancestors. Advocates claim that eliminating plant foods can reduce inflammation, promote weight loss, and improve digestion. However, it is important to note that the carnivore diet is highly restrictive and lacks the diversity of nutrients found in a balanced diet.
The Paleo Diet:
The Paleo diet seeks to emulate the eating habits of our Paleolithic ancestors. It promotes the consumption of whole, unprocessed foods such as lean meats, fish, fruits, vegetables, nuts, and seeds, while excluding grains, legumes, dairy products, and processed foods. By focusing on nutrient-dense foods and eliminating potential allergens, the Paleo diet aims to support weight loss, improve digestion, and reduce the risk of chronic diseases.
The Mediterranean Diet:
The Mediterranean diet is inspired by the traditional eating patterns of countries bordering the Mediterranean Sea. It emphasizes plant-based foods such as fruits, vegetables, whole grains, legumes, nuts, and seeds, while incorporating moderate amounts of fish, poultry, and dairy products. This approach is rich in healthy fats, antioxidants, and fiber, which have been associated with a reduced risk of heart disease, improved brain function, and overall longevity.
The Standard American Diet (S.A.D.):
The Standard American Diet, unfortunately, is characterized by a high intake of processed foods, refined sugars, unhealthy fats, and a low consumption of fruits, vegetables, and whole grains. This diet is associated with a variety of health problems, including obesity, diabetes, heart disease, and certain types of cancer. It lacks the nutrient density and balance necessary for optimal health.
Benefits of Each Approach:
Keto: Weight loss, improved insulin sensitivity, increased mental clarity, potential therapeutic benefits for epilepsy and neurological disorders.
Carnivore: Potential for reduced inflammation, weight loss, and improved digestion. However, it may lack essential nutrients and long-term sustainability.
Paleo: Improved weight management, reduced risk of chronic diseases, increased nutrient intake, elimination of potential allergens.
Mediterranean: Heart health, improved brain function, longevity, reduced risk of chronic diseases, balanced nutrient intake.
S.A.D.: No significant benefits compared to the other diets mentioned. Associated with various health issues.
Conclusion:
Choosing the right low carb diet depends on individual goals, preferences, and health considerations. While the ketogenic and carnivore diets offer unique metabolic effects, it is important to consider the
long-term sustainability and potential nutrient deficiencies. The Paleo and Mediterranean diets provide a balanced approach by emphasizing whole, unprocessed foods and diverse nutrient profiles. In contrast, the Standard American Diet (S.A.D.) is associated with numerous health problems due to its reliance on processed and unhealthy foods.
It is essential to note that individual responses to different diets may vary. What works for one person may not yield the same results for another. It is always advisable to consult with a healthcare professional or a registered dietitian before making significant dietary changes.
Ultimately, the key to a successful and sustainable low carb diet lies in finding a balance that aligns with your health goals and preferences. Incorporating whole, nutrient-dense foods while reducing processed carbohydrates can have a positive impact on weight management, overall health, and disease prevention. By understanding the variables and benefits of different low carb diets, you can make an informed decision and embark on a journey towards improved well-being.
Comparison chart highlighting the macronutrient composition of each diet:
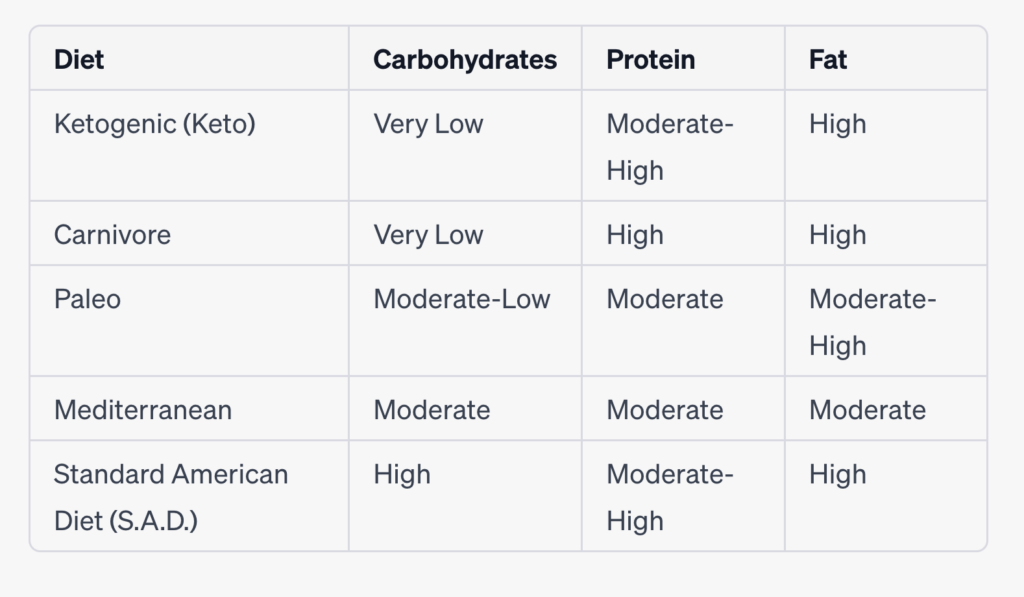
Please note that the macronutrient ratios mentioned above can vary based on individual preferences and specific interpretations of each diet. Additionally, the “Moderate” category indicates a more balanced distribution rather than being excessively high or low.
It’s important to keep in mind that macronutrient ratios can be adjusted within each diet based on individual needs, health goals, and preferences. Consulting with a healthcare professional or a registered dietitian can provide personalized guidance for determining the ideal macronutrient breakdown for your specific circumstances.
Remember that while macronutrients play a significant role in dietary choices, the quality of food, micronutrient content, and overall balance of the diet are also crucial factors to consider for long-term health and well-being.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Chicken Thighs with Mushrooms and Spinach: A Flavorful Delight
By Lisa Fitzmeyer
Introduction: Chicken thighs with mushrooms and spinach is a delicious and nutritious dish that combines tender chicken thighs, earthy mushrooms, and vibrant spinach. This recipe offers a harmonious blend of flavors and textures, making it a satisfying meal for any occasion. Follow this simple recipe to create a mouthwatering dish that will impress your family and friends.
Ingredients:
- 6-8 chicken thighs
- Avocado oil
- 2 tablespoons fresh rosemary, chopped
- 1 medium onion, sliced
- White button mushrooms, sliced
- 4 cloves of garlic, chopped
- Fresh spinach or kale
Instructions:
- Preheat the oven to 350°F (175°C).
- In a large skillet, heat 4 tablespoons of avocado oil over medium heat. Place the chicken thighs in the pan, skin side down, and cook until the skin is crispy and golden brown. This will take about 5-6 minutes. Flip the chicken thighs and cook for an additional 2-3 minutes. The chicken will not be fully cooked at this stage but will finish cooking in the oven.
- Transfer the chicken thighs to a baking dish, arranging them in a single layer.
- In the same skillet, add the sliced onions, chopped garlic, and fresh rosemary. Sauté them in the remaining oil until the onions become jammy and translucent. This process will take about 5 minutes.
- Add the sliced mushrooms to the skillet and sauté for an additional 3-4 minutes until they are tender and slightly browned.
- Spoon the onion, garlic, and mushroom mixture around the chicken thighs in the baking dish, distributing it evenly.
- Place the baking dish in the preheated oven and bake for 30 minutes, or until the chicken thighs are cooked through and reach an internal temperature of 165°F (74°C).
- Remove the baking dish from the oven and increase the oven temperature to 400°F (200°C).
- Meanwhile, coat the fresh spinach or kale with a drizzle of olive oil, ensuring that all leaves are lightly coated.
- Spread the oiled spinach or kale around the chicken and vegetables in the baking dish.
- Return the dish to the oven and bake for an additional 5 minutes, or until the spinach or kale wilts slightly.
- Carefully remove the baking dish from the oven. The chicken thighs should be juicy, and the mushrooms, onions, and spinach or kale will have melded together beautifully.
- Serve the chicken thighs with mushrooms and spinach alongside your favorite keto-friendly sides, such as roasted cauliflower or zucchini noodles, or a fresh green salad dressed with olive oil and lemon juice. Enjoy the delightful flavors and wholesome goodness of this keto-friendly chicken thighs with mushrooms and spinach dish!
Enjoy the delightful flavors and wholesome goodness of this chicken thighs with mushrooms and spinach dish!
Note: Feel free to adjust the quantities of ingredients to suit your preferences and the number of servings desired.
Rejuvenate Your Body: Harnessing the Power of Intermittent Fasting for Autophagy
By Stephen Fitzmeyer, MD
Introduction
Intermittent fasting has gained significant attention in recent years due to its potential health benefits. One of the key aspects of intermittent fasting is its ability to stimulate a process called autophagy. Autophagy, which translates to “self-eating” in Greek, is a natural cellular process that plays a vital role in maintaining cellular health and overall well-being. In this article, we will explore the importance of intermittent fasting for autophagy and how it can positively impact our health.
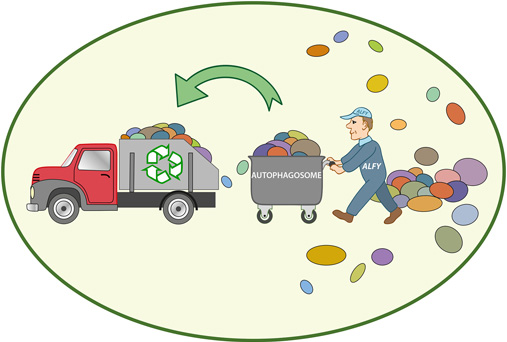
Understanding Autophagy
Autophagy is an intricate process by which cells remove and recycle damaged, dysfunctional, or unnecessary components, such as proteins and organelles. It acts as a cellular cleansing mechanism, promoting cellular renewal and enhancing the overall efficiency of our cells. This process is crucial for maintaining cellular health, preventing the accumulation of toxic substances, and reducing the risk of various diseases, including neurodegenerative conditions, cardiovascular diseases, and cancer.
Autophagy and Intermittent Fasting
Intermittent fasting is an eating pattern that cycles between periods of fasting and eating. It doesn’t focus on what you eat but rather when you eat. Commonly, individuals adopt one of the popular intermittent fasting methods, such as the 16/8 method (fasting for 16 hours and eating within an 8-hour window) or the 5:2 diet (eating normally for five days and significantly reducing calorie intake for two non-consecutive days).
Studies have suggested that intermittent fasting can stimulate autophagy, thereby enhancing cellular health. When we fast, our body experiences a drop in insulin levels, leading to a state of increased autophagy. During this fasting period, the body shifts from utilizing glucose as a primary source of energy to utilizing stored fats through a process called ketosis. Ketosis has been shown to induce autophagy and promote cellular rejuvenation.
Benefits of Autophagy
- Cellular Regeneration: Autophagy allows for the removal of damaged or malfunctioning cellular components, promoting cellular regeneration and rejuvenation. This process helps to maintain cellular health and prevent the accumulation of toxic substances that can lead to various diseases.
- Anti-Aging Effects: Autophagy has been linked to anti-aging effects. By eliminating damaged cellular components and proteins, autophagy can help slow down the aging process and delay age-related diseases.
- Disease Prevention: Autophagy plays a crucial role in protecting against various diseases, including neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, cardiovascular diseases, and certain types of cancer. By eliminating dysfunctional cells and reducing oxidative stress, autophagy helps to mitigate the risk of these diseases.
- Metabolic Health: Intermittent fasting and autophagy can have positive effects on metabolic health. It has been shown to improve insulin sensitivity, regulate blood sugar levels, and promote healthy weight management.
- Clearance of Protein Aggregates: Intermittent fasting triggers autophagy, enabling cells to remove protein aggregates, including amyloid, tau, alpha-synuclein, and Lewy bodies. These aggregates are associated with neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and other conditions. By effectively clearing these toxic substances, intermittent fasting helps decrease neuroinflammation and supports brain health.
- Reduction of Primed Glial Cells: Primed glial cells, when overactive, contribute to neuroinflammation. Intermittent fasting helps clear these primed glial cells, further decreasing neuroinflammation and offering neuroprotective effects. This reduction in neuroinflammation is key in preserving brain function and mitigating the risk of neurodegenerative diseases.
Conclusion
Autophagy is a vital cellular process that promotes cellular health, rejuvenation, and disease prevention. Intermittent fasting serves as an effective tool to stimulate autophagy and reap its numerous benefits. By adopting intermittent fasting, individuals can harness the power of autophagy, enhancing their overall well-being and reducing the risk of various age-related diseases.
However, it is important to note that intermittent fasting may not be suitable for everyone, especially those with specific medical conditions or nutritional needs. It is advisable to consult with a healthcare professional or a registered dietitian before implementing any significant dietary changes.
Incorporating intermittent fasting into one’s lifestyle, along with a balanced diet and regular exercise, can pave the way for improved cellular health and a healthier, more vibrant life.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/