- Home
- About
- Portfolio
- Crush the Match – Medical School and Residency Platform
- Food¢ense – Curbing Childhood Obesity and Food Waste
- HealthStack – Shared and Jailed HIPAA Hosting $50
- Marta Care – Let Us Help When You Can’t
- MD Idea Lab – We Build Prototypes for Doctors
- Nervcell – The Healthcare Web Browser
- Patient Keto – Personalized Keto Medicine and Telehealth
- SwipeChart – Rapid EMR Interface
- Treatment Scores – Quantifying the Science of Medicine
- Treatments – Diagnosed. Now What?
- VIDRIO – Google Glass and EMR Interface
- Blog
- Contact
Category: Biochemistry
Unveiling the Differences: The Dawn Phenomenon vs. The Somogyi Effect in Diabetes Management
By Stephen Fitzmeyer, MD
Introduction:
Diabetes management encompasses various challenges, including understanding and addressing the intricacies of blood glucose fluctuations. Two phenomena that often perplex individuals with diabetes and healthcare professionals are the dawn phenomenon and the Somogyi effect. While both involve abnormal blood glucose levels, these phenomena differ in their timing, triggers, underlying mechanisms, and management strategies. In this article, we delve into these distinctions to shed light on the unique characteristics of the dawn phenomenon and the Somogyi effect in diabetes management.
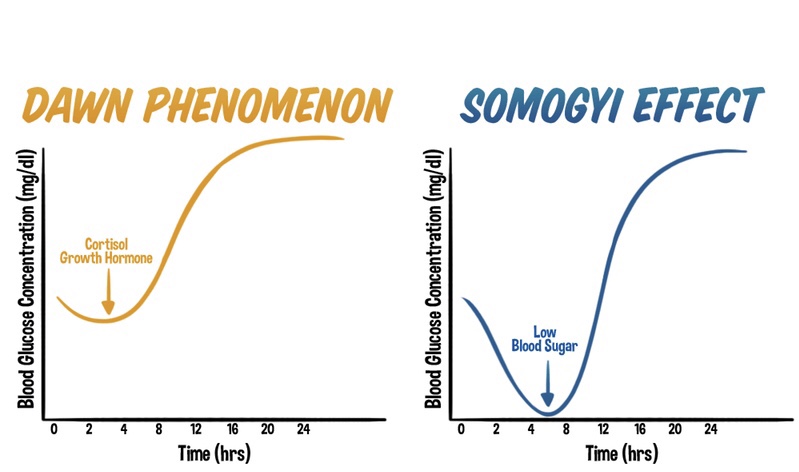
The Dawn Phenomenon: An Early Morning Rise in Blood Glucose
The dawn phenomenon is a well-known phenomenon observed in individuals with diabetes, characterized by an abnormal rise in blood glucose levels during the early morning hours, typically before waking up. Hormonal changes play a significant role in triggering this phenomenon. Increased release of hormones such as cortisol, growth hormone, and glucagon during the early morning hours leads to insulin resistance and stimulates gluconeogenesis. As a result, blood glucose levels rise without any preceding hypoglycemia.
The Somogyi Effect: Rebound Hyperglycemia Following Nocturnal Hypoglycemia
In contrast, the Somogyi effect involves a rebound hyperglycemia following a period of nocturnal hypoglycemia. This phenomenon occurs when blood glucose levels drop too low during the night, often due to excessive insulin administration or inadequate carbohydrate intake before bedtime. Nocturnal hypoglycemia triggers a counterregulatory response in the body, resulting in the release of hormones such as glucagon, cortisol, and growth hormone. These hormones stimulate gluconeogenesis and glycogenolysis, leading to a rebound rise in blood glucose levels during the morning or throughout the day.
Distinguishing Factors: Timing, Triggers, and Underlying Mechanisms
One of the primary distinctions between the dawn phenomenon and the Somogyi effect lies in their timing and triggers. The dawn phenomenon occurs during the early morning hours, driven by natural hormonal changes, while the Somogyi effect occurs as a response to nocturnal hypoglycemia.
Underlying mechanisms also differ between the two phenomena. The dawn phenomenon involves overactive gluconeogenesis as a contributing factor, as the liver produces glucose from non-carbohydrate sources. In contrast, the Somogyi effect encompasses a complex interplay of factors, including the release of counterregulatory hormones that stimulate both gluconeogenesis and glycogenolysis.
Management Strategies:
Effective management of the dawn phenomenon and the Somogyi effect requires tailored approaches based on their unique characteristics.
Managing the dawn phenomenon involves adjusting insulin regimens, specifically optimizing basal insulin doses during the early morning hours. Lifestyle modifications, including regular exercise, a balanced diet, and adequate sleep, can also aid in stabilizing blood glucose levels.
The management of the Somogyi effect requires identifying patterns of nocturnal hypoglycemia through consistent blood glucose monitoring. Adjusting insulin doses, timing, or types can prevent hypoglycemia and subsequent rebound hyperglycemia. Ensuring sufficient carbohydrate intake before bedtime and maintaining consistent sleep patterns are essential strategies in managing the Somogyi effect.
Conclusion:
Understanding the distinctions between the dawn phenomenon and the Somogyi effect is crucial in diabetes management. While both phenomena involve abnormal blood glucose fluctuations, their timing, triggers, underlying mechanisms, and management strategies differ significantly. Healthcare professionals play a vital role in recognizing these differences and tailoring individualized care plans to optimize blood glucose control. By comprehending the unique characteristics of the dawn phenomenon and the Somogyi effect, individuals with diabetes can work with their healthcare teams to effectively manage these phenomena and achieve improved overall well-being.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Rejuvenate Your Body: Harnessing the Power of Intermittent Fasting for Autophagy
By Stephen Fitzmeyer, MD
Introduction
Intermittent fasting has gained significant attention in recent years due to its potential health benefits. One of the key aspects of intermittent fasting is its ability to stimulate a process called autophagy. Autophagy, which translates to “self-eating” in Greek, is a natural cellular process that plays a vital role in maintaining cellular health and overall well-being. In this article, we will explore the importance of intermittent fasting for autophagy and how it can positively impact our health.
Understanding Autophagy
Autophagy is an intricate process by which cells remove and recycle damaged, dysfunctional, or unnecessary components, such as proteins and organelles. It acts as a cellular cleansing mechanism, promoting cellular renewal and enhancing the overall efficiency of our cells. This process is crucial for maintaining cellular health, preventing the accumulation of toxic substances, and reducing the risk of various diseases, including neurodegenerative conditions, cardiovascular diseases, and cancer.
Autophagy and Intermittent Fasting
Intermittent fasting is an eating pattern that cycles between periods of fasting and eating. It doesn’t focus on what you eat but rather when you eat. Commonly, individuals adopt one of the popular intermittent fasting methods, such as the 16/8 method (fasting for 16 hours and eating within an 8-hour window) or the 5:2 diet (eating normally for five days and significantly reducing calorie intake for two non-consecutive days).
Studies have suggested that intermittent fasting can stimulate autophagy, thereby enhancing cellular health. When we fast, our body experiences a drop in insulin levels, leading to a state of increased autophagy. During this fasting period, the body shifts from utilizing glucose as a primary source of energy to utilizing stored fats through a process called ketosis. Ketosis has been shown to induce autophagy and promote cellular rejuvenation.
Benefits of Autophagy
- Cellular Regeneration: Autophagy allows for the removal of damaged or malfunctioning cellular components, promoting cellular regeneration and rejuvenation. This process helps to maintain cellular health and prevent the accumulation of toxic substances that can lead to various diseases.
- Anti-Aging Effects: Autophagy has been linked to anti-aging effects. By eliminating damaged cellular components and proteins, autophagy can help slow down the aging process and delay age-related diseases.
- Disease Prevention: Autophagy plays a crucial role in protecting against various diseases, including neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, cardiovascular diseases, and certain types of cancer. By eliminating dysfunctional cells and reducing oxidative stress, autophagy helps to mitigate the risk of these diseases.
- Metabolic Health: Intermittent fasting and autophagy can have positive effects on metabolic health. It has been shown to improve insulin sensitivity, regulate blood sugar levels, and promote healthy weight management.
- Clearance of Protein Aggregates: Intermittent fasting triggers autophagy, enabling cells to remove protein aggregates, including amyloid, tau, alpha-synuclein, and Lewy bodies. These aggregates are associated with neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and other conditions. By effectively clearing these toxic substances, intermittent fasting helps decrease neuroinflammation and supports brain health.
- Reduction of Primed Glial Cells: Primed glial cells, when overactive, contribute to neuroinflammation. Intermittent fasting helps clear these primed glial cells, further decreasing neuroinflammation and offering neuroprotective effects. This reduction in neuroinflammation is key in preserving brain function and mitigating the risk of neurodegenerative diseases.
Conclusion
Autophagy is a vital cellular process that promotes cellular health, rejuvenation, and disease prevention. Intermittent fasting serves as an effective tool to stimulate autophagy and reap its numerous benefits. By adopting intermittent fasting, individuals can harness the power of autophagy, enhancing their overall well-being and reducing the risk of various age-related diseases.
However, it is important to note that intermittent fasting may not be suitable for everyone, especially those with specific medical conditions or nutritional needs. It is advisable to consult with a healthcare professional or a registered dietitian before implementing any significant dietary changes.
Incorporating intermittent fasting into one’s lifestyle, along with a balanced diet and regular exercise, can pave the way for improved cellular health and a healthier, more vibrant life.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
Unveiling the Role of ApoB and the Therapeutic Potential of Ketogenic Lifestyle and Intermittent Fasting in Atherosclerosis
By Stephen Fitzmeyer, MD
Introduction:
Atherosclerosis, a major contributor to cardiovascular disease, arises from a complex interplay of various factors. Among them, Apolipoprotein B (ApoB) emerges as the primary driver in the development and progression of this condition. In this article, we delve into the critical role of ApoB in atherosclerosis and shed light on the influence of inflammation in enhancing its effects.
Understanding the Role of ApoB:
ApoB, a protein found in lipoproteins such as low-density lipoprotein (LDL) particles, serves as a key player in atherosclerosis. It acts as a carrier, facilitating the transportation of cholesterol to peripheral tissues, including the arterial walls. In the absence of ApoB, the initiation and progression of atherosclerosis are virtually non-existent.
The Significance of ApoB in Atherosclerosis:
ApoB takes center stage in atherosclerosis, as it is responsible for delivering cholesterol-rich lipoproteins, particularly LDL, to arterial walls. These lipoproteins undergo modifications and become trapped in the arterial intima, initiating the formation of fatty streaks. With time, inflammation is triggered, attracting immune cells and accelerating the transformation of fatty streaks into advanced atherosclerotic plaques.
Inflammation and its Role:
While inflammation is a key player in atherosclerosis, it acts as an enhancer rather than the primary driver. Inflammation exacerbates the process by promoting the retention and modification of ApoB-containing lipoproteins, leading to plaque progression and instability. Thus, controlling inflammation becomes crucial in managing atherosclerosis, but addressing the root cause—ApoB—remains essential.
Implications and Therapeutic Strategies:
Understanding the central role of ApoB opens up avenues for therapeutic interventions in managing atherosclerosis. Addressing ApoB levels and reducing the burden of cholesterol-rich lipoproteins is key. Here, lifestyle modifications such as adopting a low-carbohydrate, high-fat diet (such as a ketogenic diet) and implementing intermittent fasting can prove beneficial. These approaches help regulate ApoB-containing lipoproteins, mitigate their retention in arterial walls, and slow down the progression of atherosclerosis.
Furthermore, lifestyle modifications that target additional risk factors associated with atherosclerosis, such as hypertension and obesity, should be considered. Engaging in regular physical activity, maintaining a healthy weight, and managing other comorbidities can complement the efforts to address ApoB and reduce the overall risk of atherosclerosis.
Conclusion:
ApoB stands as the primary driver in the initiation and progression of atherosclerosis, while inflammation serves to enhance and accelerate the process. Recognizing the pivotal role of ApoB provides insights into therapeutic strategies that can mitigate its effects. By adopting lifestyle modifications, such as a low-carbohydrate, high-fat diet and intermittent fasting, individuals can positively influence ApoB levels and manage atherosclerosis. Combining these interventions with measures to address other risk factors offers a comprehensive approach to reducing the burden of atherosclerosis and promoting cardiovascular health.
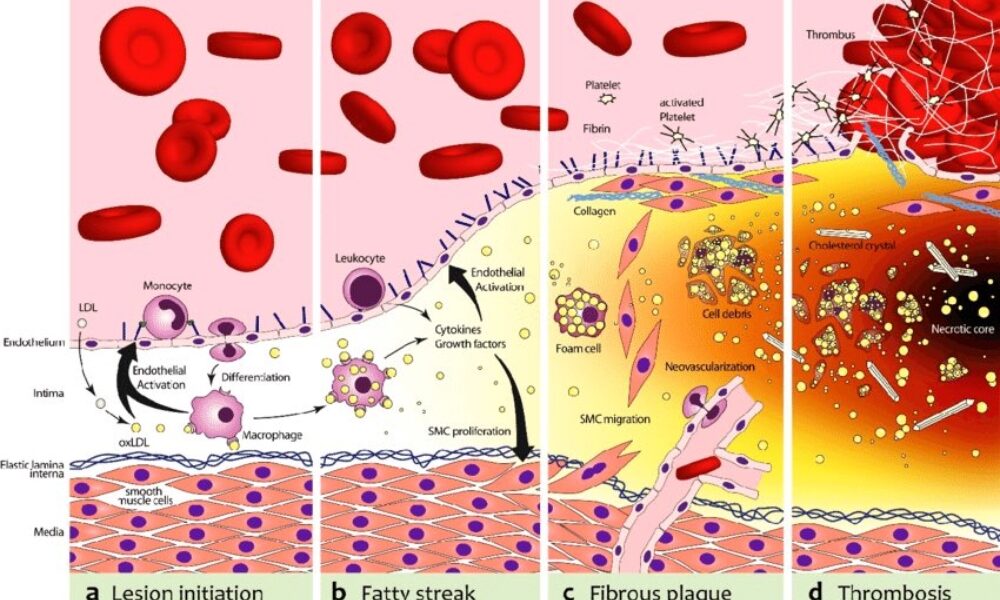
The biochemical pathway of plaque formation involving ApoB can be described as follows:
- ApoB synthesis: ApoB is a protein synthesized in the liver and intestines. It is a major component of very low-density lipoprotein (VLDL) and LDL particles.
- Lipoprotein assembly: VLDL particles are assembled in the liver and contain ApoB-100. They transport triglycerides and cholesterol from the liver to peripheral tissues. During circulation, VLDL particles undergo enzymatic changes, resulting in the conversion of triglycerides into free fatty acids and glycerol.
- LDL formation: As VLDL particles lose triglycerides, they become smaller and denser, transforming into LDL particles. LDL contains a single molecule of ApoB-100 and is the primary carrier of cholesterol in the bloodstream.
- LDL uptake: LDL particles bind to LDL receptors on cell surfaces, allowing the cells to take up cholesterol. These receptors are present in various tissues, including the arterial walls.
- Retention and modification: In the arterial walls, LDL particles can undergo modifications, such as oxidation and glycation, making them more prone to retention. These modified LDL particles interact with extracellular matrix proteins and proteoglycans in the arterial intima, leading to their entrapment within the vessel walls.
- Inflammation and foam cell formation: The retained LDL particles, along with their cholesterol content, trigger an inflammatory response. Immune cells, particularly macrophages, migrate to the site of inflammation. They engulf the cholesterol-rich LDL particles, transforming into foam cells, which are characterized by their lipid-filled cytoplasm.
- Fatty streak formation: The accumulation of foam cells and other immune cells results in the formation of fatty streaks, which are the initial visible signs of plaque development. Fatty streaks consist of foam cells, lipids, inflammatory cells, and smooth muscle cells.
- Advanced plaque formation: Over time, the fatty streaks can progress into more advanced atherosclerotic plaques. These plaques are characterized by a fibrous cap composed of smooth muscle cells and collagen, a lipid-rich core containing foam cells and cholesterol, and a necrotic center.
Throughout this biochemical pathway, ApoB plays a crucial role in the transport of cholesterol to peripheral tissues, including the arterial walls. It facilitates the delivery of cholesterol-rich LDL particles, which, under certain conditions, contribute to the formation of atherosclerotic plaques. Understanding this pathway provides valuable insights into potential therapeutic targets for preventing and managing plaque formation and related cardiovascular diseases.
Author: Stephen Fitzmeyer, M.D.
Physician Informaticist
Founder of Patient Keto
Founder of Warp Core Health
Founder of Jax Code Academy, jaxcode.com
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/