- Home
- About
- Portfolio
Crush the Match – Medical School and Residency Platform
Food¢ense – Curbing Childhood Obesity and Food Waste
HealthStack – Shared and Jailed HIPAA Hosting $50
Marta Care – Let Us Help When You Can’t
MD Idea Lab – We Build Prototypes for Doctors
Nervcell – The Healthcare Web Browser
Patient Keto – Personalized Keto Medicine and Telehealth
SwipeChart – Rapid EMR Interface
Treatment Scores – Quantifying the Science of Medicine
Treatments – Diagnosed. Now What?
VIDRIO – Google Glass and EMR Interface
- Blog
- Contact
Mitochondria: The Powerhouses that Prefer Fats over Sugars
Introduction:
Mitochondria, the tiny organelles found within our cells, play a crucial role in energy production. They are responsible for converting the food we consume into usable energy in the form of ATP (adenosine triphosphate). While both fats and sugars can serve as fuel sources for our mitochondria, growing evidence suggests that these cellular powerhouses have a preference for utilizing fats as their primary energy substrate. In this article, we will explore the reasons why mitochondria favor fats over sugars for efficient energy production.
- The Efficiency of Fat Oxidation:
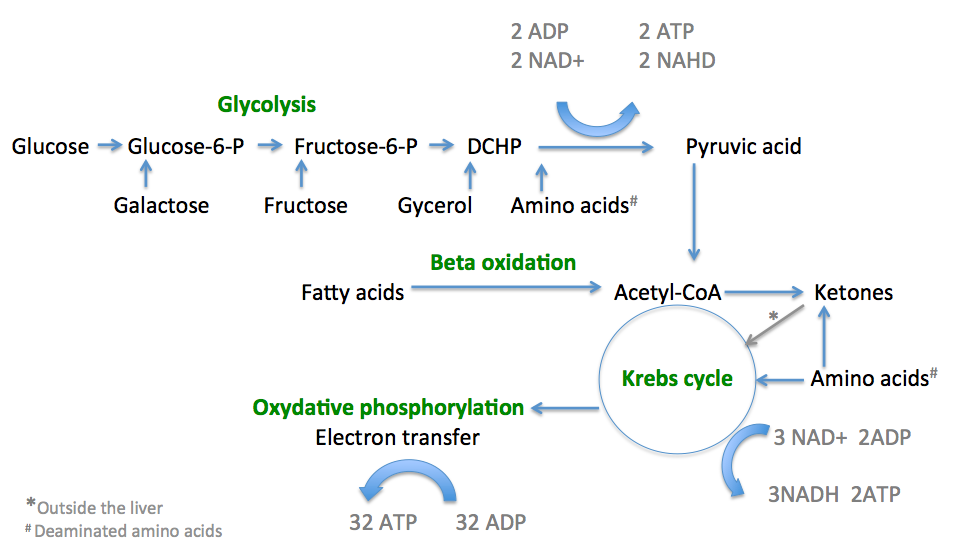
Mitochondria exhibit a remarkable efficiency in oxidizing fats compared to sugars. When fatty acids enter the mitochondria, they undergo beta-oxidation, a process that breaks down long-chain fatty acids into smaller units called acetyl-CoA. This acetyl-CoA then enters the citric acid cycle, where it is further metabolized to produce energy-rich molecules such as NADH and FADH2. These molecules are critical for ATP synthesis through oxidative phosphorylation.
The process of fat oxidation generates a greater yield of ATP per molecule compared to the metabolism of sugars. Fats contain more carbon atoms and provide a dense source of energy, supplying a sustained and long-lasting fuel for our cells.
- Mitochondrial Adaptation to Fat Metabolism:
The mitochondria are highly adaptable organelles that can adjust their metabolic machinery based on the available fuel sources. When the body predominantly relies on fats for energy, such as during periods of fasting or a low-carbohydrate diet, mitochondria undergo a process called metabolic flexibility or metabolic switching. This adaptation enables the mitochondria to enhance their capacity to metabolize fats efficiently.
Through increased expression of enzymes and transporters involved in fat metabolism, mitochondria become better equipped to handle fatty acids, ensuring a continuous supply of energy. This adaptation leads to increased mitochondrial biogenesis, the formation of new mitochondria, and improved oxidative capacity, ultimately enhancing fat oxidation as the preferred fuel source.
- Reduced Oxidative Stress and Mitochondrial Health:
Mitochondria have a lower propensity to generate harmful byproducts, such as reactive oxygen species (ROS), during fat oxidation compared to sugar metabolism. When glucose is metabolized, it can result in a higher production of ROS, which can contribute to oxidative stress and damage to cellular components, including mitochondria themselves.
By primarily utilizing fats as a fuel source, mitochondria can reduce the generation of ROS and maintain a healthier environment. This helps to preserve mitochondrial function and integrity, supporting overall cellular health and longevity.
Conclusion:
Mitochondria, the powerhouses of our cells, exhibit a clear preference for utilizing fats over sugars for energy production. The efficiency of fat oxidation, the adaptability of mitochondria to fat metabolism, and the reduced oxidative stress associated with fat utilization all contribute to this preference. By prioritizing fats as a fuel source through dietary choices or strategies like intermittent fasting or a low-carbohydrate diet, we can optimize mitochondrial function and support overall cellular health.
However, it’s important to highlight the remarkable benefits of a ketogenic diet in achieving optimal energy production and overall well-being. While carbohydrates have their role in providing quick bursts of energy and supporting specific bodily functions, a ketogenic diet, which prioritizes fats as the primary fuel source, can have profound effects on our health. By entering a state of ketosis, where the body relies on fat metabolism and produces ketones as an alternative energy source, individuals can experience enhanced fat burning, improved mental clarity, reduced inflammation, and stable energy levels throughout the day. Understanding individual needs and goals can guide the establishment of an optimal macronutrient ratio, allowing individuals to harness the power of ketosis and support their cellular powerhouses in achieving peak performance and well-being.
Author: Dr. Stephen Fitzmeyer, M.D.
Physician Informaticist and Founder of Warp Core Health
Connect with Dr. Stephen Fitzmeyer:
Twitter: @PatientKeto
LinkedIn: linkedin.com/in/sfitzmeyer/
- Hoppel, C. (2003). The Role of Carnitine in Normal and Altered Fatty Acid Metabolism. American Journal of Kidney Diseases, 41(4), S4-S12. doi: 10.1016/s0272-6386(03)00003-5
- Kelley, D. E., & Mandarino, L. J. (2000). Fuel Selection in Human Skeletal Muscle in Insulin Resistance: A Replication. Diabetes, 49(5), 677-683. doi: 10.2337/diabetes.49.5.677
- Mootha, V. K., et al. (2003). PGC-1α-responsive Genes Involved in Oxidative Phosphorylation are Coordinately Downregulated in Human Diabetes. Nature Genetics, 34(3), 267-273. doi: 10.1038/ng1180
- Schooneman, M. G., et al. (2013). Fatty Acid Partitioning in the Energetics of the Liver: An Overview and Update. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids, 1831(12), 169-176. doi: 10.1016/j.bbalip.2013.03.013
- Storlien, L. H., et al. (2004). Adaptation of Muscle Mitochondria to Prolonged Exercise Training. Experimental Physiology, 89(1), 13-22. doi: 10.1113/expphysiol.2003.026203
- Veech, R. L. (2004). The Therapeutic Implications of Ketone Bodies: The Effects of Ketone Bodies in Pathological Conditions: Ketosis, Ketogenic Diet, Redox States, Insulin Resistance, and Mitochondrial Metabolism. Prostaglandins, Leukotrienes and Essential Fatty Acids, 70(3), 309-319. doi: 10.1016/j.plefa.2003.09.007